The measurement of radiation is crucial in various fields, from healthcare to nuclear physics. This article delves into the intricate world of radiation measurements, exploring units, the concept of half-life, and methods for measuring radiation doses. By understanding these aspects, we can effectively quantify and manage the impact of radiation on humans and the environment.
Units of Radiation Measurement
Radiation is measured using various units tailored to different types and energies of radiation. These units provide a standardized way to quantify the amount of radiation exposure.
1. Activity: Becquerel (Bq)
The Becquerel (Bq) measures the rate at which a radioactive substance decays. One Becquerel is one decay event per second. It’s the fundamental unit of activity in the International System of Units (SI).
2. Exposure: Coulomb per kilogram (C/kg)
Exposure measures the ionization of air due to radiation. It’s used in fields like radiology. The older unit, the Roentgen (R), is still used in the United States.
3. Absorbed Dose: Gray (Gy)
The Gray (Gy) measures the energy absorbed by matter due to radiation. It quantifies the amount of energy deposited per unit mass. One Gray is equal to one joule of energy absorbed by one kilogram of matter.
4. Equivalent Dose: Sievert (Sv)
The Sievert (Sv) takes into account the biological effects of different types of radiation. It’s the absorbed dose multiplied by a quality factor that reflects the radiation’s relative biological effectiveness.
5. Effective Dose: Sievert (Sv)
Effective dose considers both the type of radiation and the tissue’s sensitivity. It’s used to assess potential health risks.
Half-Life: A Measure of Radioactive Decay
Radioactive decay is a natural process that transforms unstable atomic nuclei into more stable configurations, releasing energy in the form of radiation. The rate at which this decay occurs is a fundamental property of each radioactive substance. To quantitatively describe this rate, scientists use the concept of half-life.
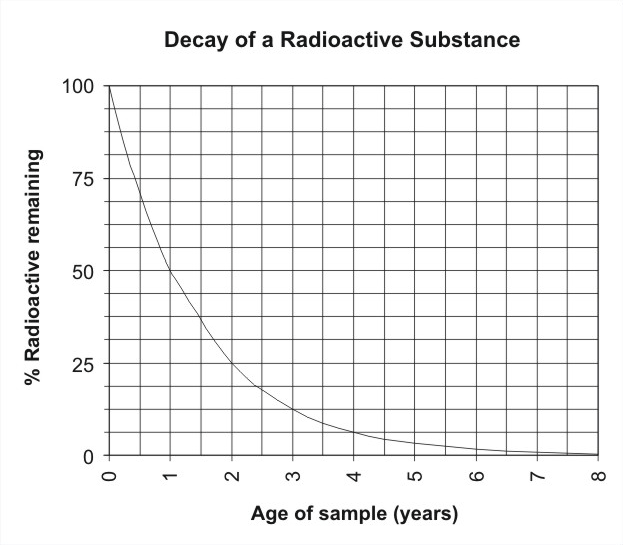
Understanding Radioactive Decay
Radioactive isotopes are characterized by an unstable nucleus that seeks to achieve a more stable state. As a result, these isotopes undergo a process of decay, during which they emit radiation and transform into different elements. This transformation is random and unpredictable at the individual level but follows a statistically predictable pattern when observed across a large number of atoms.
The Concept of Half-Life
The half-life of a radioactive substance is the time it takes for half of the atoms in a given sample to decay. It’s a fundamental property of the isotope, much like its atomic mass or charge. Half-life is denoted by the symbol “t½” and is usually expressed in units of time, such as seconds, years, or even millennia.
Imagine you have a sample of a radioactive substance. At the start, there are a certain number of radioactive atoms in the sample. As time passes, these atoms undergo decay, transforming into other elements and releasing radiation. After one half-life, half of the initial radioactive atoms will have decayed, and the other half will remain. Similarly, after two half-lives, only a quarter of the initial atoms will remain radioactive, and so on.
Variability of Half-Life
Different radioactive isotopes have different half-lives, ranging from fractions of a second to billions of years. This variability allows scientists to categorize isotopes into short-lived and long-lived ones, depending on their decay rates.
Practical Significance of Half-Life
The concept of half-life has numerous practical applications across various fields:
- Radiometric Dating: Archaeologists and geologists use the concept of half-life to determine the ages of rocks and artifacts. By measuring the ratio of parent isotopes to daughter isotopes in a sample, they can calculate how many half-lives have passed and thus determine the sample’s age.
- Medical Imaging and Treatment: In medical fields, isotopes with specific half-lives are used for imaging and treatment purposes. For instance, technetium-99m, with a half-life of about 6 hours, is commonly used in nuclear medicine for diagnostic imaging.
- Environmental Studies: The half-lives of isotopes released into the environment due to nuclear accidents or industrial activities help predict the persistence of radioactive pollutants and their potential impact on ecosystems and human health.
- Nuclear Energy: Understanding the half-lives of isotopes is crucial for the safe handling and storage of nuclear waste. Different isotopes have different decay rates, which impact the duration of radioactive hazards.
- Astrophysics: The study of half-lives provides insights into the nuclear processes occurring within stars, which influence their lifecycles, energy output, and eventual fate.
Radiation Dose Measurement
Radiation dose measurement is crucial for assessing health risks and ensuring safety in various applications.
1. Personal Dosimeters
Dosimeters are worn by workers exposed to radiation. They measure personal dose equivalent and help monitor safe exposure levels.
2. Geiger-Muller Counters
Geiger-Muller counters detect ionizing radiation by measuring the number of ion pairs produced. They’re widely used in radiation monitoring.
3. Scintillation Detectors
Scintillation detectors measure radiation by detecting the light emitted when ionizing radiation interacts with certain materials.
4. Film Badges
Film badges contain photographic film sensitive to radiation. After a period of exposure, the film is developed, and its darkness indicates the dose received.
5. TLD (Thermoluminescent Dosimetry)
TLD uses crystals that emit light when heated after radiation exposure. The intensity of emitted light corresponds to the absorbed dose.
You May Also Like
